Dilatometer was designed in ILTPE NASU so that allows precision measurements of the linear
coefficient of thermal expansion in studied temperature range. Measurements of thermal expansion
provide information about the specific phase transitions and quantum phenomena in solids
[Low Temp. Phys.42, 788 (2016)]. Low-temperature dilatometry for studying the thermal expansion of solids in the
temperature range 2 - 290 K is shown in Fig. above.
Sorption of gas impurities in nanomaterials
Sorption and desorption of gas impurities by powders of nanomaterials are investigated
in the temperature interval 2 - 290 K that allows to analyse the thermally activated
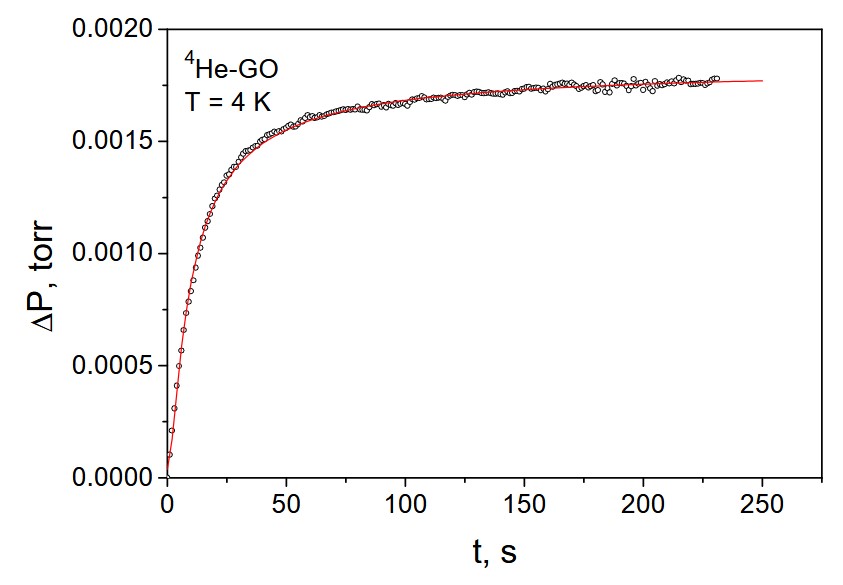
and tunneling mechanisms of sorption. The example of the pressure variations in the
process of 4He desorption from the sample of graphene oxide is shown on Fig. below
[Low Temp. Phys. 39, 1090 (2013)].
The group maintains a continuous scientific information exchange with researchers
in different countries, including Instituto de Carboquímica (Zaragoza, Spain) and
Lappeenranta-Lahti University of Technology LUT (Finland).
On cooperation bases with the Department of Experimental
Physics Umea University (Sweden), Australian Nuclear Science and Technology
Organization (Australia), National Kharkov Physico-Technical Institute Scientific
Center (Ukraine) complex investigations have been performed on fullerite C60
(pure and doped with various gases). A programme has been developed to investigate
the physical properties of C60 jointly with the National Mirzo Ulugbek University
of Uzbekistan. The investigations of the thermal properties of SWNTs have been
performed with E. L. Andronikashvili Institute of Physics Georgian Academy of Sciences,
Tbilisi (Georgia).
Some principal results obtained by Dilatometric group:
The researchers of this group have studied the thermal expansion of solidified gases
Ar, Kr, Ne, H2, HD, D2, CH4, CHD3, CD4,
N2, CO2, CO, N2O, NH3, CCl4, CBr4, SF6
and some of their solid solutions. Also, the thermal expansion of fullerite, various structural materials and crystal
for low temperature and space applications has been investigated. The negative thermal expansion
and the phenomenon of the orientational polyamorphism of the fullerite C60 have been found out at
temperatures of liquid helium at last years. Low temperature dilatometric investigations of the
thermal expansion were performed for the first time on samples of pure and doped with various
gases single-walled carbonnanotube bundles (SWNTs) in the direction perpendicular to the bundle
axes. The impurity and quantum effects in thermal expansion of carbon nanostructures have been
investigated. Quantum diffusion of 4He, H2 and Ne in fullerite C60
has been detected and investigated.
It has been found that the spatial 4He and 3He redistribution in bundles of carbon nanotubes is of
the tunnel character. The effect of radioactive irradiation of bundles of nanotubes with γ-quanta in
the atmosphere of various gases upon the radial thermal expansion of nanotube bundles and their
sorption of hydrogen has been investigated for the first time experimentally. It is found that
irradiation of the samples has caused a drastic increase in the quantity of the hydrogen chemosorbed
by the nanotubes. Sorption and the subsequent desorption of 4Не, Н2, Ne, N2,
CH4 and Kr gas impurities by graphene oxide (GO), glucose-reduced GO (RGO-Gl) and hydrazine-reduced GO (RGO-Hz)
powders have been investigated in the temperature interval 2 - 290 K. The influence of reduction
temperatures on the structure and the sorption capacity of thermally reduced graphene (TRGO) has
been investigated systematically. Most of the experimental results have been included into the
handbooks and monographs published in the USSR (before 1990 year), Ukraine and the USA.
Prof. Alexander Dolbin is a laureate of the State Prize in Science and Technology in
2011 for the series of works “Quantum effects and structural self-organization in new multifunctional
nanomaterials”.
In 2022, he received the Award of the National Academy of Sciences of Ukraine "For Professional
Achievements".